Avalon BioMedical (Shenzhen) Limited is a subsidiary of Avalon BioMedical (Management) Limited, a Hong Kong biotechnology incubator focused on delivering next generation healthcare solutions to benefit humanity. The Shenzhen branch dedicates to the design, development, manufacture and distribution of phototherapy medical devices.
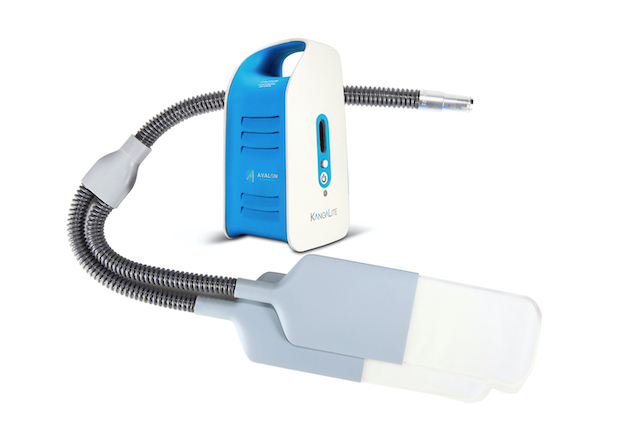
Our first product is a LED-based wearable phototherapy device (Kanga-care) for the treatment of neonatal jaundice. It was developed by a team of pediatric specialists, engineers, and scientists. Kanga-care was approved as a Class II medical device in China in 2018. The second generation, KangaLite, featured a redesigned light emitting panels and swaddle for reducing weight and improving the product usability. KangaLite has received the approval from the China’s NMPA (formally known as CFDA), CE certificate for EU regions, and 510(k) clearance from the US FDA.
 Neonatal Phototherapy Device
Neonatal Phototherapy Device